Cancer therapies are a complex problem that scientists are working to solve. Immunotherapy is a common treatment that includes manipulating genes or administering glycolytic metabolites, small molecules, that improve the immune system’s response to cancer. Yet, gene therapy for already sick patients is intricate, and metabolites often diffuse prematurely and struggle to reach their targets.
One strategy involves blocking glycolysis, an essential process which produces energy using glucose to inhibit cancer cell growth. However, this approach affects immune cells, which also rely on glycolysis for energy. Immune cells such as dendritic cells (DCs) allow T cells, “soldiers” of the immune system, to recognize melanoma tumours (skin cancer), leading to their elimination. Therefore, researchers need to find a way to increase glycolysis only in DCs and not in the cancer cells.
The researchers have formulated a vaccine, known as F16BP microparticle (F16BP MP) that includes three main components: F16BP, poly(IC), and either a melanoma antigen or mRNA. F16BP is a product made during glycolysis, allowing the process to continue within DCs, while poly(IC) enhances the efficacy of vaccines. The use of mRNA allows for versatile treatment options. In addition to F16BP MP, a cancer cell glycolysis-inhibitor drug, PFK15, is administered.
F16BP MP was designed to be phagocytosed or “eaten” by DCs to ensure that F16BP release is only done by DCs and not the cancer cells. This allows DCs to have enough energy to target the cancer cells. F16BP MP released metabolites for up to 6 hours when tested in the lab under conditions equivalent to the human system. Although this is a desired effect as DCs have a lifespan of 1-3 days after phagocytosis, the drugs must be administered every other day.
In a study using mouse models with highly aggressive melanoma tumours, F16BP MP-PFK15 was administered every other day for 60 days to evaluate its effectiveness. The vaccinated mice showed a slowed tumour growth rate and an increased survival rate of around 25 days compared to the untreated group. After treatment, testing found that T cells and DCs still functioned normally.
Furthermore, to increase treatment possibilities, DCs were adoptively transferred and effected to another strain of tumour. This method involved the isolation of DCs, treatment with only F16BP, and then infusion into the bloodstream along with PFK15 injections. The survival of the mice increased by around ten days of a 30-day experiment. Additionally, tumour growth was slowed, with the treatment group showing the smallest tumour size by day 16.
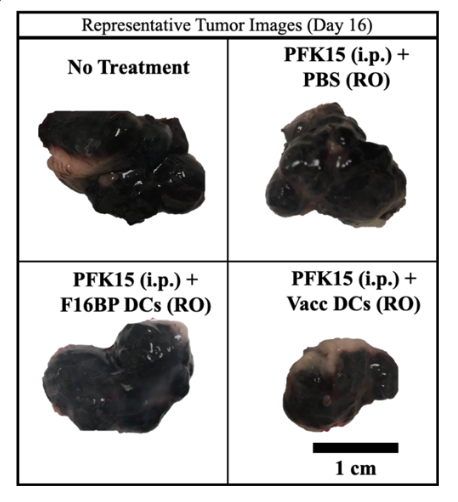
In all the methods tested above, treatment groups showed an elevated glycolysis rate in DCs and a strong immune response against the cancer cells. The entire F16BP MP-PFK15 system must be present for these results to be achieved. Furthermore, it was found that a higher dosage of the system enhanced results in all margins tested. Remarkably, the survival rate of an increased dosage was 100% in all experiments, but further dosing tests were not conducted.
The research’s versatility is a strong suit in cancer therapy advancement. Exploration of different treatment administration methods is crucial for the variable immune responses between individuals in therapy. Successful inhibition of another strain of tumour gives rise to the possibility of eradicating tumours with further trials. It has been shown that increased antigen and poly(IC) in the vaccines or increased injections in adoptively transferred DCs is promising.
To target specific types of cancer, exploring alternative essential pathways in cancer cells may be necessary. It may also be possible for different types of immune cells to be rescued, or even multiple types simultaneously.
Fascinatingly, this research is the first time immune cells are rescued while inhibiting the same pathways used by the cancer cells. These ground-breaking findings could be the gateway to the advancement of cancer immunotherapy.
References:
Inamdar, S., Suresh, A. P., Mangal, J. L., Ng, N. D., Sundem, A., Wu, C., Lintecum, K., Thumsi, A., Khodaei, T., Halim, M., Appel, N., Jaggarapu, M. M., Esrafili, A., Yaron, J. R., Curtis, M., & Acharya, A. P. (2023). Rescue of dendritic cells from glycolysis inhibition improves cancer immunotherapy in mice. Nature Communications, 14(1). https://doi.org/10.1038/s41467-023-41016-z