
Imagine skipping your morning coffee, the feel of sluggish, slow and barely moving. That’s what muscles feel like without its morning dystrophin. MyoAAV-UV is a perfect cup for utrophin, perking the muscles back up and keeping them running.
Back ground
As DMD is the most common childhood genetic disorder, and it affects 1 in every 3500- 5000 male infants. These babies are lack of dystrophin, which is a type of protein that is essential for muscle strength and stability which typically causes them to suffer from walking, then wheelchair dependent and ultimately facing fatal cardiorespiratory failure. Yet even there are different promising ways to help them, due to issues like limited patient coverage, suboptimal therapeutic efficacy, high cost and immunogenicity issues have prevented a fully effective cure.
Utrophoin as a protein is very similar to dystrophin, and by activating the expression of utrophin from the patient’s genome it can compensate for the loss of dystrophin and improve muscle functions.
Yet there is an issue, AAV, the cup that delivers utrophin, has limited capacity thus it requires multiple doses or cups to deliver all necessary components. To conquer that Research in China Kunming university of science and technology propose that MyoAAV-UA can use a compacted CRISPR activation system that can fit in a single AAV vector. By doing so it not only simplifies delivery but also improves its targeting precision and reduce off target effects and immune response.
Research
Researchers divided the mics into 3 groups, one as healthy mice, one as DMD model, and there others are treated by MyoAAV-UA. The researchers inject the MyoAAV-UA to the mouse and allow it to travel through the bloodstream and get into the muscle cells. Then collect muscle samples that include biceps and diaphragms, 8 weeks and 6 months after injection to compare the differences between groups.
Once MyoAAV-UA gets inside the cell it releases its genetic cargo into the nucleus. Then the muscle specific promoter activates expression of a compact CRISPR activator that is programmed to target the promoter region of the utrophin gene (UTRN). This activator binds to the utrophin promoter and calls for the cell’s own transcription machinery to enhance the gene’s natural activity. By doing so, the muscle cells can produce large amounts of full length utrophin protein that can restore cellular stability and function that were lost due to the lack of dystrophin.
Results
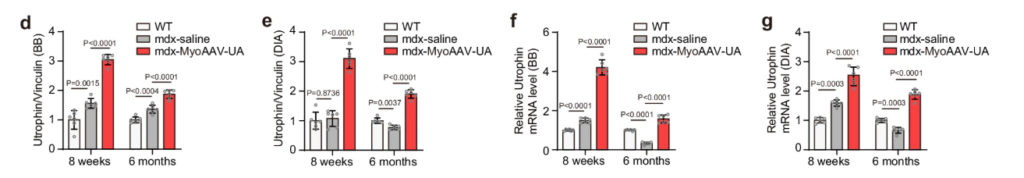
The results were stunning. After treatment the utrophin protein levels showed about 200% in research at 8 weeks and remained around 90% higher at 6 months in both biceps and diaphragm compared to healthy mice which is shown in figure d and e. At the mRNA level, Utrophin expression increased by about 320% in the biceps and 200% in the diaphragm at 8 weeks, then stabilized to around 60% to 90% above normal levels at 6 months which is shown in figure f and g. This suggests MyoAAV-UA does can successfully activate Utrophin and last for more than 6 months
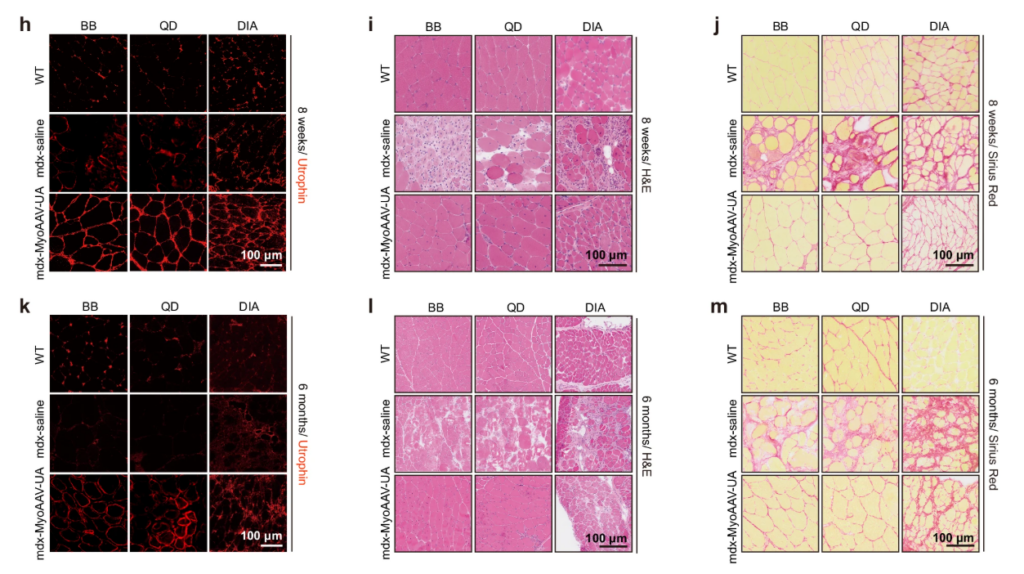
The researcher also found out utrophin is located on the sarcolemma which is the cell membrane and where dystrophin normally sits, and an improvement on muscle structure. Histological analysis revealed an improvement by showing fewer centrally nucleated fiber which indicates it has reduced ongoing regeneration (figures h and k), and lower inflammation (figures i and l), and reduced in fibrosis or scar tissue formation as shown by Sirius RED staining (figures j and m).
What’s next
Although it’s still in the pre-clinical stage, the results are promising. As more and more testing of safety, dosage and long term effect on larger animal models before human trials, there’s growing hope that one day DMD can be cured or at least treated far more effectively.
Citation
Wu, R., Li, P., Xiao, P. et al. Activation of endogenous full-length utrophin by MyoAAV-UA as a therapeutic approach for Duchenne muscular dystrophy. Nat Commun 16, 2398 (2025). https://doi.org/10.1038/s41467-025-57831-5






