
Imagine a world where we can slow down or even stop the progression of Alzheimer’s disease. A novel therapeutic peptide, RI-AG03, is showing promise in the fight against this devastating neurodegenerative condition by targeting a critical factor: tau protein aggregation. Let’s take a closer look at what makes RI-AG03 a potential game-changer in the field of Alzheimer’s research.
The Problem with Tau Aggregation
Alzheimer’s disease is marked by the presence of neurofibrillary tangles composed of tau protein. In healthy neurons, tau helps stabilize microtubules, but in Alzheimer’s, tau becomes abnormally phosphorylated and starts to aggregate, forming clumps that disrupt cell function. These aggregates are thought to be highly toxic, leading to neuron death and cognitive decline.
Despite extensive research, targeting tau aggregation has remained challenging, as most treatments have focused primarily on managing symptoms rather than addressing the underlying pathology.
The need for a treatment that targets tau aggregation directly is more pressing than ever. Current therapies that target beta-amyloid plaques have shown limited success in halting disease progression, which underscores the importance of focusing on tau. By understanding how tau aggregation contributes to neuronal dysfunction and ultimately cognitive decline, researchers hope to develop a therapy that could modify the disease course. This is where RI-AG03 comes into play—a novel therapeutic approach designed to directly inhibit tau aggregation and potentially alter the progression of Alzheimer’s disease.
Introducing RI-AG03: A Novel Peptide Inhibitor
Researchers recently developed RI-AG03, a peptide designed to inhibit tau aggregation in a highly specific manner. Unlike other aggregation inhibitors, RI-AG03 works by targeting two critical aggregation hotspots in the tau protein—the 306VQIVYK311 and 275VQIINK280 sequences. This precise targeting was made possible through Fmoc solid-phase peptide synthesis (SPPS), a technique that allowed scientists to build RI-AG03 step-by-step with high fidelity, ensuring the correct sequence and incorporation of D-amino acids for increased stability. The specificity of RI-AG03 allows it to effectively neutralize the tau aggregation process while minimizing off-target effects that have been an issue with previous treatments.
How Does RI-AG03 Work?
RI-AG03 doesn’t merely cap tau proteins to prevent aggregation. Instead, it redirects tau into forming large, amorphous aggregates that have reduced beta-sheet content, a structural feature often associated with the toxicity of protein aggregates. By doing so, RI-AG03 limits the formation of smaller, more harmful oligomers, which are typically the most neurotoxic forms of tau. The formation of these non-toxic aggregates offers a safer route for tau to exist without damaging neurons. In experiments, RI-AG03 exhibited remarkable efficacy in reducing tau aggregation. It showed a half-maximal inhibitory concentration (IC50) of 7.83 µM for TauΔ1-250 and 5 µM for Tau2N4R, demonstrating that it can inhibit multiple tau isoforms effectively. This means that RI-AG03 can be effective across different forms of tau aggregation, which is critical for treating diverse tauopathies.
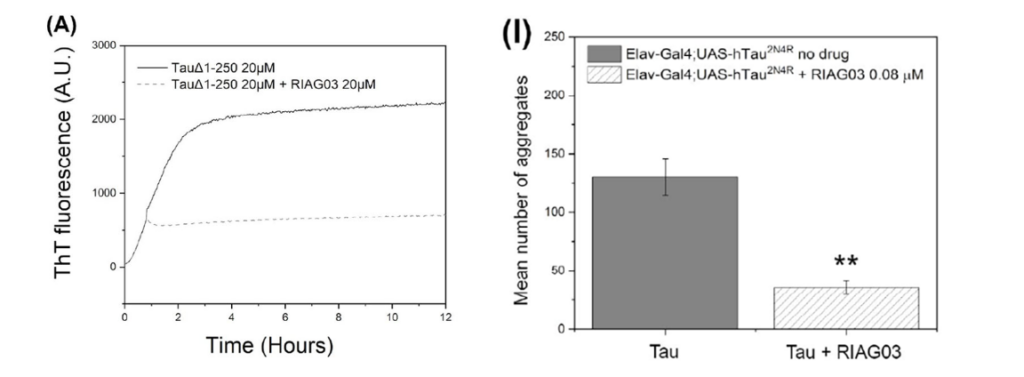
Figure 1. (A) Thioflavin T (ThT) fluorescence assay showing the effect of RI-AG03 on tau aggregation over time. The solid line represents tau aggregation without RI-AG03 and the dashed line represents tau treated with RI-AG03. (I) The effect of RI-AG03 on the number of tau aggregates in Drosophila expressing human tau. The bar on the left (solid) shows the mean number of aggregates without RI-AG03 treatment, while the bar on the right (striped) shows a significant reduction in aggregates with 0.08 µM RI-AG03 treatment. The double asterisk (**) indicates a statistically significant decrease in tau aggregates compared to the untreated group.
In Vitro and In Vivo Breakthroughs
In Vitro Success
To better understand and target this aggregation process, Thioflavin-T (ThT) fluorescence assays were utilized. ThT specifically binds to β-sheet-rich structures, enabling researchers to monitor the formation and inhibition of tau aggregates in real-time. This was crucial for observing whether RI-AG03 could effectively inhibit tau aggregation under controlled experimental conditions.
To validate its potential, RI-AG03 was tested both in vitro and in vivo. In vitro experiments utilized ThT fluorescence to monitor the aggregation process (Figure 1A1). Stable ThT readings, as shown in Figure 1A1, indicated that RI-AG03 effectively prevented tau aggregation rather than breaking down tau clumps that had already formed. When RI-AG03 is added, the fluorescence intensity does not increase significantly over time, suggesting that the formation of new aggregates is being inhibited. The peptide’s purity and structure were confirmed using mass spectrometry, including MALDI-TOF and Quadrupole Electrospray MS, to ensure that the synthesized product met experimental standards and was ready for biological testing.
In Vivo Success
Perhaps even more compelling was the success seen in Drosophila (fruit fly) models of tauopathy. Flies overexpressing human tau typically develop a “rough-eye” phenotype, characterized by disrupted eye morphology due to neurodegeneration. Treatment with RI-AG03 led to visible improvements in eye morphology, reducing aggregation-related defects. Flies treated with RI-AG03 also showed increased survival, further highlighting its therapeutic potential. Figure 1I1 shows a clear reduction in tau aggregates in the Drosophila model after RI-AG03 treatment. This graph effectively illustrates how RI-AG03 reduces tau aggregates in living organisms, as treated flies displayed significantly fewer aggregates compared to untreated ones, highlighting its potential in reducing tau toxicity.
Why RI-AG03 Matters for Alzheimer’s Treatment
The potential of RI-AG03 lies in its dual-targeting capability and its ability to encourage tau proteins to aggregate into a less toxic form. Unlike other small-molecule inhibitors, RI-AG03 targets specific regions on the tau protein, reducing the likelihood of unwanted side effects. Moreover, being a D-amino acid peptide, RI-AG03 is highly stable and can cross biological barriers, making it a feasible candidate for future clinical applications.
Current Alzheimer’s therapies focus mostly on symptoms rather than targeting the underlying causes. RI-AG03’s ability to modify the aggregation pathway of tau offers a potential disease-modifying approach that could change the course of Alzheimer’s and other tauopathies. By altering how tau aggregates, RI-AG03 aims to directly reduce neurotoxicity and provide a protective effect for neurons, offering patients hope for a future with fewer symptoms and slower progression. This could mark a significant shift in how Alzheimer’s is treated, moving away from purely symptomatic relief toward addressing one of the core mechanisms driving neurodegeneration.
What’s Next?
RI-AG03 is still in the experimental phase, but its development is a significant milestone in the fight against tauopathies. As researchers work to validate its effects in more complex models, including mammalian systems, RI-AG03 could eventually pave the way for clinical trials and, hopefully, a new generation of effective treatments for Alzheimer’s disease.
With the advancements being made in tau-targeting therapies like RI-AG03, the future of Alzheimer’s treatment looks a little brighter. This peptide-based inhibitor could be the key to unlocking a new era of precision medicine, one where therapies are designed to directly counteract the mechanisms driving neurodegeneration.
References
- Aggidis, A.; Devitt, G.; Zhang, Y.; Chatterjee, S.; Townsend, D.; Fullwood, N. J.; Ortega, E. R.; Tarutani, A.; Hasegawa, M.; Cooper, A.; Williamson, P.; Mendoza‐Oliva, A.; Diamond, M. I.; Mudher, A.; Allsop, D. A Novel Peptide‐Based Tau Aggregation Inhibitor as a Potential Therapeutic for Alzheimer’s Disease and Other Tauopathies. Alzheimer’s & Dementia 2024. https://doi.org/10.1002/alz.14246.








