Candida auris is an emerging multidrug-resistant, life-threatening fungus that kills within the clinical setting. The exact mortality rate of these opportunistic, vulture-like infections approaches 30%. This frightening approximation is only exacerbated by an increasing resistance to echinocandins, the “front-line” and most-responsive antifungal medication for the species (Doorley et al., 2025; Toda et al., 2019). The trench-bound treatment is a predominantly fungicidal (fungus-killing) intravenous sapper, stopping fungal cell wall formation.
Unlike other strains such as C. albicans, a leading cause of candidemia, where the fungal infection spreads through the bloodstream like a metastasising cancer and can result in widespread organ failure, C. auris is indisputably more resistant to azoles, a staple suppressor of fungal growth. Meanwhile, a coinflip estimates your odds of effective treatment with the other relevant antifungal drug, amphotericin B, with C. auris resistance rates recently rising to nearly 50%. (Rybak et al., 2022). In exhausting this list of agents, we arrive at the problem. If C. auris adds echinocandins to its list of subjugated antifungal agents, we’ll be out our last, consistent, cure.
Directly determining how this antifungal resistance arose is a major challenge. As the authors of the study state, there is a “lack of available molecular tools” suitable for delving into the specifics of C. auris (Doorley et al., 2025). Existing methods require recreations of mutations in related fungal organisms or lab-grown strains, fostered in the presence of antifungals. Both approaches invite confounding variables, the third wheel of cause and effect relationships, to the table (Tulchinsky & Varavikova, 2015, p. 116). When a third party is involved, the couple’s true dynamic is disguised. Perhaps it’s the result of the sterility of the environment, or the fact that the couple is “out of the house,” so to speak. Regardless, the significance of this, when it comes to an argument (or a war) we can’t risk losing, the importance is apparent.
To help prevent finality from creeping into the fight against C. auris, researchers from St. Jude’s Children’s Hospital’s Department of Pharmacy and Pharmaceutical Sciences have developed a gene-editing system called “episomal plasmid-induced Cas9” (EPIC) optimised to determine the impact of the S639F mutation on C. auris on antifungal resistance (Doorley et al., 2025).
Diving deeper into the case study, a mutation called S639F, which occurs in FKS1 in C. auris, was the subject of interrogation. Already fingered as the culprit behind echinocandin resistance in another species, C. albicans, the mutation affects the gene that codes for an enzyme that synthesises a vital component of the fungal cell wall, β-glucan. β-glucan is the collagen, calcium, and potassium of Candida cells’ “bones,” and it makes up the majority of their cell structure. Without it, cell wall integrity and the cell become the target of the discriminatory practices of physics—differences in pressure cause fluids to rush in, bursting the cell like a housing bubble.
Returning to the EPIC system, how was it made?
It starts with the industry standard for gene-editing, CRISPR, which cuts targeted stretches of Nucleotides. Nucleotides, the building blocks for cellular code sequences, are the victim of the S639F mutation, in which a single nucleotide for FKS1 coding is replaced.
Then, a C. albicans-specific vector was translated across species. This was achieved by stripping out and replacing the C. albicans-wired sequences with functionally-identical sequences from C. auris. Following this, the template responsible for the S639F mutation was created. Finally, the rehashed vector and S639F mutation sequence were introduced into C. auris via a fungal plasmid. This introduction came in the form of an offer the cell couldn’t refuse. Imagine it awaking dazed, thoroughly dehydrated, and situated deep within the Atacama desert. You offer it a glass of water, or a slow death in the inhospitable conditions you grew it in. By accepting this “water” ( the plasmid), it has invited the whole EPIC system in.
Following this system incorporation, testing of the four facets of EPIC function began.
First, nucleotide sequencing confirmed that the S639F mutation was retained in the cell’s offspring. Then, complete genome analysis verified that the EPIC system only produced the S639F mutation. Next, they tested EPIC on clinical strains of C. auris. Another mutation was chosen and edited into five hospital-bound strains. The mutation was present in 45/50 sequenced cells, while the remaining five produced unintended changes (Figure 1B).
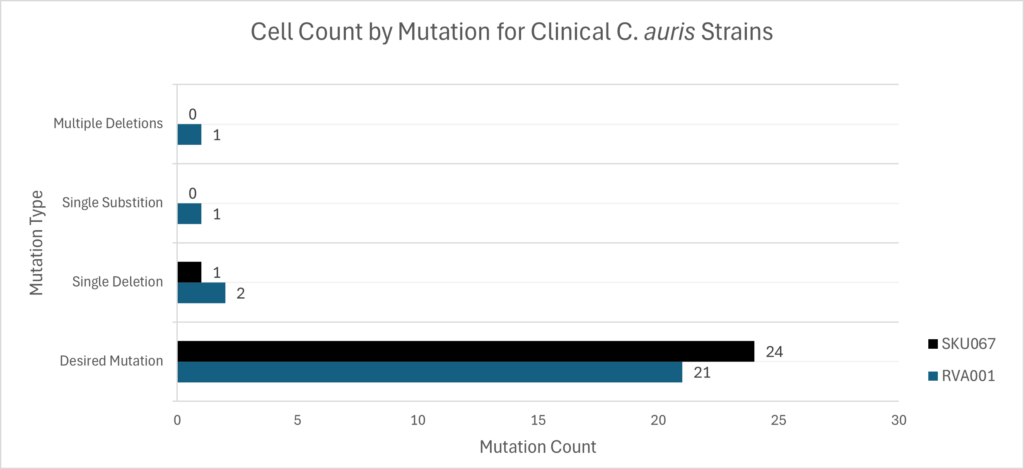
Note. Figure adapted from “A Candidozyma (Candida) auris–Optimized Episomal Plasmid–Induced Cas9-Editing System Reveals the Direct Impact of the S639F-Encoding FKS1 Mutation,” by L. A. Doorley, V. Meza-Perez, S. J. Jones, & J. M. Rybak, 2025, Journal of Infectious Diseases, 232(3), e529–e536 (https://doi.org/10.1093/infdis/jiaf285). CC BY-NC-ND 4.0.*
Finally, they confirmed that the C. auris-optimised EPIC system edited only single nucleotides. Another two clinically sourced C. auris strains received two types of edits. The former was the encoding mutation (S639F), while the latter was intended to have no effect (WTs). The four strains were then sequenced, and in the majority, only the desired nucleotide was edited (Doorley et al., 2025).
At last, we arrive at our mutation interrogation by the comparison of resistance to echinocandins in the strains developed above. The intent: defining the impact of the S639F mutation on antifungal resistance. This impact quickly becomes visually apparent.
If you follow the Xs, which denote the point at which 50% of fungal growth was inhibited, you can point out the resistance induced by the S639F mutation. The two S639F strains persisted in even a two-hundred-and-fifty-six-fold increase in micafungin concentration (Figure 2). Of particular importance is the mutation’s facilitation of micafungin and rezafungin growth past the inhibitory concentration level that deems them effective for use in hospitals in the first place. The S639F mutations were always 20% steps ahead. This is the raw, unfiltered evidence that the EPIC-editing system promises.
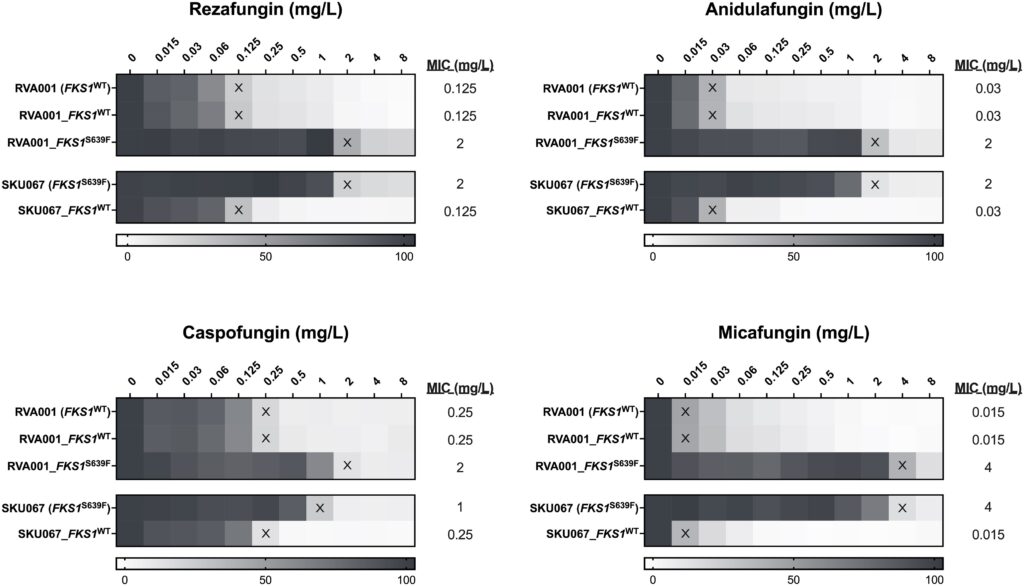
Note. Figure reproduced from “A Candidozyma (Candida) auris–Optimized Episomal Plasmid–Induced Cas9-Editing System Reveals the Direct Impact of the S639F-Encoding FKS1 Mutation,” by L. A. Doorley, V. Meza-Perez, S. J. Jones, & J. M. Rybak, 2025, Journal of Infectious Diseases, 232(3), e529–e536 (https://doi.org/10.1093/infdis/jiaf285). CC BY-NC-ND 4.0.*
By effectively introducing, facilitating, and implicating the S639F mutation in the case of echinocandin resistance, the C. auris-optimised EPIC-editing system has cleared the pathway to establishing that this mutation is a source of echinocandin resistance; one so effective that it invalidates the use of half of the available echinocandins, rezafungin and micafungin in the clinical setting.
Limitations
The small sample size of C. auris-optimised, EPIC-edited strains could obscure recurrent statistically significant editing-errors. This is particularly relevant to the single-nucleotide targeting trial, where one of four strains presented with another nucleotide edited. Additionally, only two mutations were investigated and integrated across a limited range of C. auris strains, leaving a multitude of technical challenges, applications, and trials unexplored.
References
Doorley, L. A., Meza-Perez, V., Jones, S. J., & Rybak, J. M. (2025). A Candidozyma (Candida) auris–Optimized Episomal Plasmid–Induced Cas9-Editing System Reveals the Direct Impact of the S639F-Encoding FKS1 Mutation . The Journal of Infectious Diseases, 232(3), e529–e536. https://doi.org/10.1093/infdis/jiaf285
Lombardi, L., Oliveira-Pacheco, J., & Butler, G. (2019). Plasmid-Based CRISPR-Cas9 Gene Editing in Multiple Candida Species. mSphere, 4(2), e00125-19. https://doi.org/10.1128/mSphere.00125-19
Toda, M., Williams, S. R., Berkow, E. L., Farley, M. M., Harrison, L. H., Bonner, L., Marceaux, K. M., Hollick, R., Zhang, A. Y., Schaffner, W., Lockhart, S. R., Jackson, B. R., & Vallabhaneni, S. (2019). Population-Based Active Surveillance for Culture-Confirmed Candidemia – Four Sites, United States, 2012-2016. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C. : 2002), 68(8), 1–15. https://doi.org/10.15585/mmwr.ss6808a1
Tulchinsky, T. H., & Varavikova, E. A. (2015). Measuring, Monitoring, and Evaluating the Health of a Population. In The New Public Health (3e ed., pp. 116–118). essay, Academic Press.
Rybak JM, Barker KS, Muñoz JF, et al. In vivo emergence of high-level resistance during treatment reveals the first identified mechanism of amphotericin B resistance in Candida auris. Clin Microbiol Infect. 2022;28(6):838-843. doi:10.1016/j.cmi.2021.11.024
AI assistance was used in the creation of figure citations