The COVID-19 pandemic is officially over, but the SARS-CoV-2 virus still exists. The dominant strain of the SARS-CoV-2 virus, known as the Omicron variant, continues to circulate globally, and has mutated into various sub-variants. Several particular subvariants have risen recently, with resistance to the neutralizing antibodies from previous vaccinations; in order to solve this issue, a nasal vaccine is key.
Most current SARS-CoV-2 vaccines are injected, and prevent symptomatic infection as well as more severe infections of COVID-19. These may not prevent the transmission of SARS-CoV-2, as they do not effectively eradicate the virus found in the nose and mucus. This can result in milder infections that, nonetheless, can still spread the virus. There have been efforts to develop an intranasal (through the nose) vaccine, with ten candidates under current clinical investigation, but none currently approved by the World Health Organization. Additionally, many of these intranasal vaccine candidates are based on pre-Omicron variants, and likely would not work against the newer variants.
The Study
Quick background: a widely used method for intranasal delivery is known as the subunit protein-based vaccine platform; in simpler terms, a vaccine that uses specific proteins from a virus to train the immune system, instead of the whole virus. A modified virus used to deliver genetic materials into cells, also known as a “vector”, can be used to deliver these proteins.
In this study, scientists developed a two-component vaccine. The first component is a modified adenovirus vector that expresses the spike protein of a variant of the SARS-CoV-2 virus; the spike protein being the virus’s protein that allows it to pierce a human cell. The second component is a trimeric – composed of three parts – lab-engineered protein that simulates the shape of the virus’s spike.
Vaccines aim to strengthen the body’s natural defences; the vaccine developed in this study aimed to induce higher concentrations of antibodies than a single component vaccine, using the two-component vaccine. Additionally a three-component vaccine, with an added spike protein to create broad-spectrum immune response; this produced similar results to the two-component vaccine, which is what we’ll be focusing on. This study was carried out through testing on an intranasal vaccination into mice, and subsequent analysis of various antibody and cell presences through methods ranging from measuring the absorbance of light through samples, to analyzing characteristics of cells as they pass through a laser. An additional trial was performed on humans, yielding similar results. Some results can be seen on the figure below:
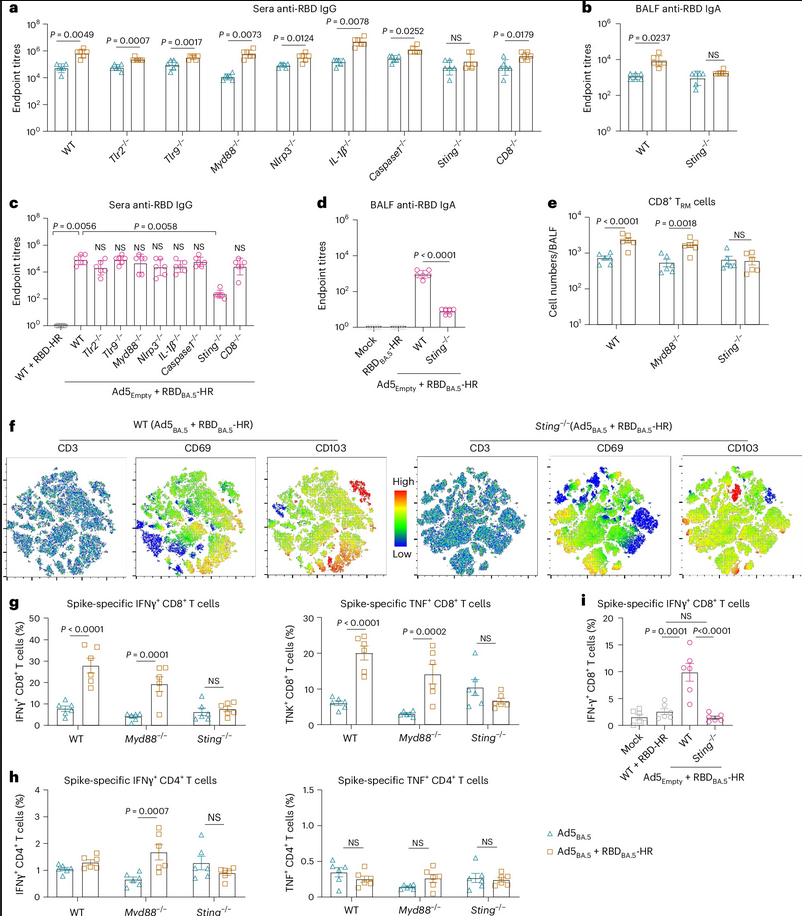
In Figures a) and b), graphs show the concentrations of antibodies in serum and lung samples from a normal mouse, as well as several strains of genetically modified mice. The blue and orange bars represent if the mice were vaccinated with only the adenovirus vector component vaccine, or vaccinated with both the adenovirus vector and the spike protein. As shown in the graphs, in every type of mouse, antibody concentrations were higher in the mouse dosed with the two-component vaccine.
Figure c) showcases the antibody concentration of mice vaccinated with the two-component vaccine against a control group that was dosed with a naked version of the modified protein. Figure d) shows similar data to a) and b). Figure e) shows the absolute number of a specific immune cell that encountered the virus. Figure f) shows a heat concentration map – similar to what you’d see in a population heat map – of mature T cells in the lung fluid from several two-component vaccinated mice. Figures g) and h) show the percentages of spike-specific T cells. Figure i) shows the percentage of a cell producing the T cells in lungs from two-component vaccinated mice.
In summary of the multiple graphs in this figure; almost universally through the mice dosed, it was found that the two-component vaccine provoked a larger immune response than the mice vaccinated with only one component of the vaccine.
Results
The results of this figure are consistent with the results of the study overall. It was found that the two-component intranasal vaccine enhanced mucosal immune responses, creating higher concentrations of antigens, as well as enhancing frequencies and neutralizing capacity of T cells, a type of white blood cell crucial to the immune system. The two-component vaccine was effective in protecting mice against a highly contagious Omicron sub-variant, and completely blocked the airborne transmission of another Omicron sub-variant. Similarly, in humans who received the vaccine, immune responses were found to be heightened. Similar results were observed in the three-component vaccine group, with, again, similar strengthened immune systems.
Although there was no statistically significant difference in the amount of respiratory virus load in mice between the two-component and single adenovirus component, hamsters did show lower viral loads between the single and double-component vaccination. In all samples from the two-component group, there were no detectable levels of the viral indicator in comparison to the single component adenovirus vaccinated group. This is further proof that the multiple-component vaccination is more effective for inducing immune responses and reducing virus spread.
To recap this entire article: current SARS-CoV-2 vaccines fail to induce complete immune reactions in the nasal area, which can lead to spread of the virus even if symptoms are decreased. To combat this, a nasal vaccine was developed using a two-component system; an adenovirus vector carried a modified spike protein derived from the Omicron variants, and a lab-modified trimeric spike protein. This induced a greater immune response in mice models than a single component vaccine, with no detectable levels of the viral indicator in comparison. A three-component vaccine with an additional modified spike protein created a similar, but broad-spectrum immune-boosting effect. This could be a path to COVID-19 vaccines of greater efficiency, to help manage the virus as it exists in our world today.
Citations
Bleier, B. S., Ramanathan Jr, M., & Lane, A. P. (2021). COVID-19 Vaccines May Not Prevent Nasal SARS-CoV-2 Infection and Asymptomatic Transmission. Otolaryngology–Head and Neck Surgery, 164(2), 305–307. https://doi.org/10.1177/0194599820982633
Hong, W., Cheng, P., Yang, J., Shi, H., Wang, Z., Li, J., Lei, H., Peng, D., He, C., Ren, W., Pan, X., Huang, Y., Alu, A., Qin, F., Wang, B., Zhou, Y., Yang, Y., Yu, W., Tang, C., … Wei, X. (2025). Intranasal vaccine combining adenovirus and trimeric subunit protein provides superior immunity against SARS-CoV-2 Omicron variant. Nature Biomedical Engineering, 1–19. https://doi.org/10.1038/s41551-025-01517-2
Photo by cottonbro studio from Pexels: https://www.pexels.com/photo/people-holding-mask-over-a-sculpture-3952229/
No AI was used in the writing or editing of this article.








