When we think about clean energy, the first things that come to our mind are solar power, wind power, hydropower and such, but never in our head will connect this with stomachs of animals. Yet, in an innovative twist, scientists have found their way to the digestive systems of herbivores, where microbes break down tough plant materials, and discover something that one day could power our world with renewable, cheap and clean energy.
Background
Saccharomyces cerevisiae, you may have not heard that name before but if I say brewer’s yeast, does that ring a bell at all? Yes, it is the ordinary yeast that we use daily. Famous for how it converts sugar into alcohol, we use it in baking, brewing, and winemaking. Beyond the kitchen, it has also helped us to power our industry, producing enzymes, vitamins, and even medicines. Recently, this little mighty guy is back in the spotlight. But this time, it’s not another way to use it in the kitchen, rather it steps onto a new stage of biofuel production.
Brewer’s yeast has been utilized by the industry for the process of converting plant sugars to ethanol, a renewable and cleaner alternative to the limiting fossil fuels. However, one of its limiting factors were that it cannot ferment xylose, which is a type of monosaccharide (sugar) mainly found in plant waste. Although there were earlier attempts in engineering brewer’s yeast to overcome this limiting factor, most of them end in failure. Only a few notables successes such as Thermus thermophilus, Piromyces sp. E2, and Orpinomyces sp. These advances proved that yeast can indeed be modified to ferment xylose, but they all fell short in efficiency. Thus till this day, we still use brewer’s yeast primarily for fermenting glucose rather than xylose. The problem is that glucose are only found in live food crops like corn, potatoes, wheats, sugarcane, etc, which means that currently, our biofuel is solely dependent on edible food. This threatens food security and harms our ecosystems. Sounds like not a fair trade off because it defeats the sole purpose of biofuel, which is to create a more sustainable future. Without a method to convert xylose into a usable form, we are leaving tons of potential energy on the table.
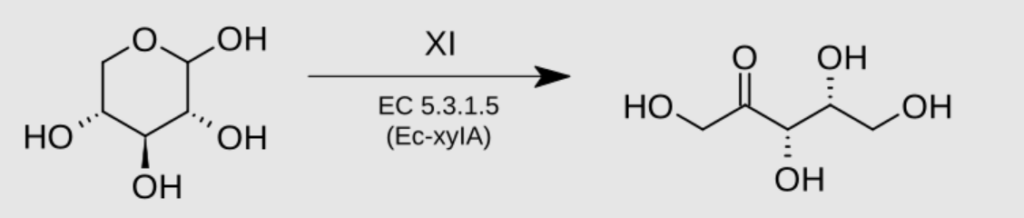
Figure 2
Xylose conversion into xylulose by xylose isomerase
To break down xylose, it has to be first converted into xylulose by an enzyme called xylose isomerase (XI), then finally into ethanol. Saccharomyces cerevisiae lacks this enzyme. Until recently, a team of scientists in Brazil successfully found the right XI genes and engineered it into Saccharomyces cerevisiae. Finally helped it to overcome the limiting factor and enable it to also have the ability to convert xylose into xylulose, then ethanol.
Methodology
To unlock the yeast’s ability to ferment xylose, the team start off their search in a weird place, the digestive systems of herbivores, specifically rumen of cows, camels, sheep, moose and even manatees. They first used HMMER V3, a software to analyze the metagenomic and metatranscriptomic dataset of these herbivores. The metagenomic dataset lets the scientists search through the DNA and all the microbes inside the rumen, while the metatranscriptomic dataset focuses on which genes were actually active while xylose were being broken down. Through this, the scientists were able to filter through all the genes and pinpoint seven potential genes that could encode XI enzymes for the yeast.
These genes were then inserted into plasmids of Escherichia coli (E.coli), which carry and multiply (clone) the plasmids. Standard miniprep protocol was performed to extract and purify these plasmids. Purified plasmids were then inserted into two strains of Saccharomyces cerevisiae , GGY018 and BVY271 via lithium acetate transformation to test the of XI genes expression. Finally, through the techniques of CRISPR-Cas9, XI genes were directly inserted into the yeast genome for stable gene expression during cell division. Polymerase chain reactions (PCR) were used to verify gene placement and correct insertion.
Now, the yeasts can produce the new XI enzyme. These modified yeast strains were grown on selective media to see if the new XI genes actually worked under selective conditions. They were grown on nutrients minimal (YNBX) and rich (YPX) media. Scientists then measured cell growth, xylose consumption, ethanol yield, and the formation of byproducts to evaluate the performance of the modified yeasts. High performance liquid chromatography was used to quantify xylose and ethanol concentrations and spectrophotometry was used to track cell growth. These data were then used to assess the efficiency of the fermentation.
Results
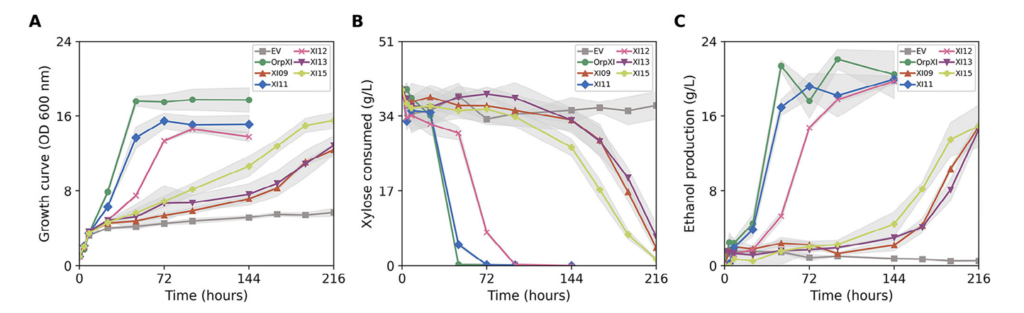
Figure 3
Figure 6 from the research article, illustrates cell growth, xylose consumed and ethanol yield compared to the control group.
In their results, these modified Saccharomyces cerevisiae with XI genes from rumen microbes showed remarkable improvements in xylose fermentation efficiency. Especially the, XI gene from sheep (XI11 and XI12) yielded the highest amount of ethanol (theoretical yield of about 90%!), while keeping the formation of byproducts at the minimum. It was also able to consume majority of the xylose in around 72 and 96 hours, which showcased its efficiency. Figure above showed that the growth rates and ethanol productivity both increased compared to the control strains that lacked the XI genes (EV). The figure also illustrated the result being comparable to Orpinomyces sp, mentioned in the introduction, it served as a benchmark enzyme (OrpXI). Additionally, the scientist later added iron into the medium and it surprisingly showed improvement in the already great efficiency of these new XIs. All in all, these results are very promising and marked a big step forward toward a more efficient biofuel production.
Next step?
The journey isn’t over yet. Although it has proved its potential in the lab, questions regarding its long-term stability and large scale productivity still remain. The next step lies in scaling these modified yeast to industrial-scale fermentations where these little guys will be put under the test of real world conditions. Achieving success here means that the smallest organisms on Earth are leading the biggest leap towards a more sustainable future. These little guys may once again change our lives, just like they did thousands of years ago with bread and wine.
References
Beatriz, et al. “Engineering Saccharomyces Cerevisiae with Novel Functional Xylose Isomerases from Rumen Microbiota for Enhanced Biofuel Production.” Authorea (Authorea), 3 Mar. 2025, https://doi.org/10.22541/au.174100785.54855403/v1. Accessed 20 Oct. 2025.








