“You have breast cancer.” Four words that thousands hear each day, and yet they never lose their weight. So what if I told you the possible key to treating one of the most stubborn cancers lies within a simple carbon–nitrogen bond?
The Problem
Breast Cancer is one of the most common forms of cancer, taking up 12% of all cancer diagnoses worldwide. Triple-negative breast cancer (TNBC), has a bleak prognosis, with an overall survival average of 11-13 months. Most breast cancers can be treated by targeting estrogen, progesterone, and HER2 receptors (the three ‘locks’) with drugs that act as ‘keys’. However, in TNBC, all three locks are missing, leaving researchers searching for a new key. Fortunately, the rise of a new compound may lead to some long needed answers.
The Solution
Schiff bases, a large group of compounds characterized by the carbon and nitrogen double bond, have become a recent research focus due to its pharmacology effects. For most healthy cells, a self destruct button is available in the form of cell apoptosis. Schiff bases have the ability to bond with metal ions and form metal complexes which can cause cell apoptosis in cancer cells. Now the real question is, could we use these schiff bases to fight TNBC? A recent study put the schiff base, β-diiminato compound to the test.
Research Findings
To start out, the β-diiminato compound had different concentrations of it inoculated for 24 hours in three types of cells: two breast cancer cell lines (MDA-MB-231 and MCF-7) and one normal liver cell (WRL-6). They then used a test called MTT assay, which involved a yellow salt that is broken down by active living cells. The researchers did this by adding MTT dye to each of the plates, then covering it and inoculating it for another 2 hours. After which they used colorimetry analysis which measures color changes to detect the presence or concentration of a substance. In this case, to measure how many cells were still alive.
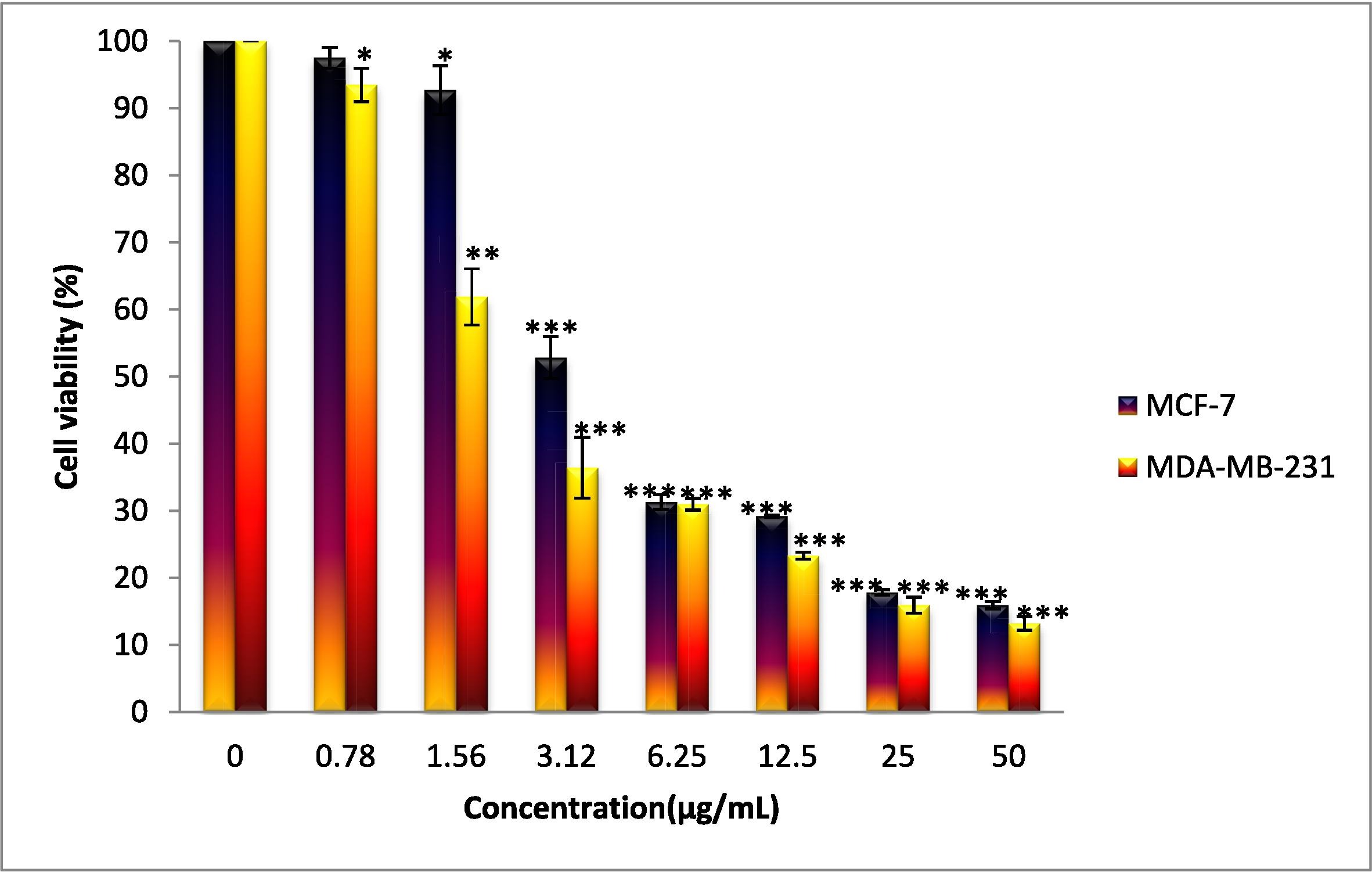
Since MDA-MB-231 represents a model of late-stage triple-negative breast cancer, further experiments focused on this cell line. Continual testing using Tryptan blue staining and LDH cytotoxicity assay confirmed the same pattern in cell survival. This confirmed that the β-diiminato compound does cause cell death in the cancer cell lines but not in liver cells.
Now researchers wanted to understand how and when the β-diiminato compound caused the effect. To do this, they used propidium iodide (PI) staining, which bonds to DNA of dead cells. The samples were then analyzed through flow cytometry, basically an x-ray for your cells. The cells are first suspended in a fluid and pass through a laser single-file. The process causes cells to scatter the light and also causes any fluorescent molecules to shine. This information is then collected and analyzed to gain understanding on many characteristics of cells. The testing revealed how the β-diiminato compound influences cell cycle progression by looking at the different concentrations of DNA throughout the cell cycle.
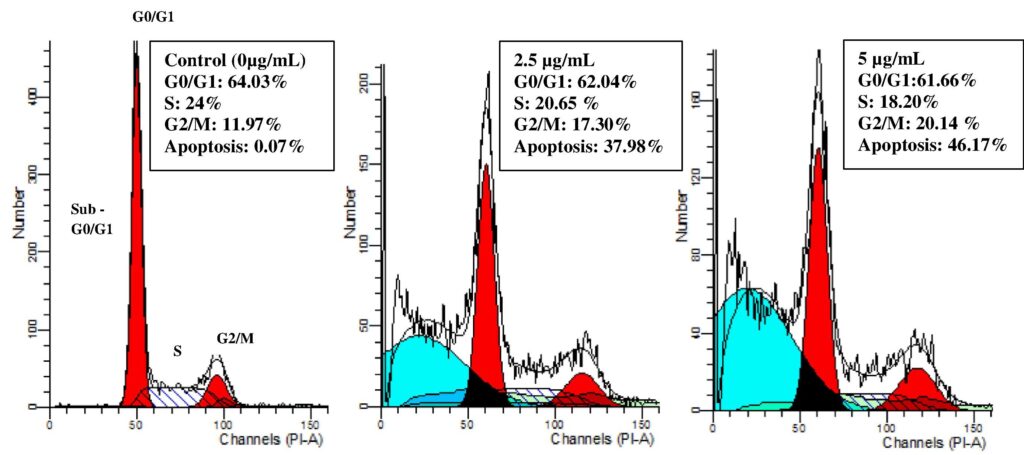
Since this study showed possible cell apoptosis, further studies included Annoxin V staining which is a dye that bonds to cells in the early stages of apoptosis. Flow cytometry analysis confirmed the previous finding, showing that the cytotoxic effects of β-diiminato were caused by apoptosis.
During the apoptosis process, many characteristics can be observed as evidence. A decrease of mitochondrial membrane potential (MMP) is one of the triggering steps of apoptosis. In addition, reactive oxygen species (ROS) are produced during apoptosis and can further promote cell death.
To better learn how apoptosis was occurring JC-1 assay and Cellular Reactive Oxygen Species Detection Assay Kit were used to measure the MMP and ROS of the cell and observed a MMP potential loss and a ROS increase respectively. This signifies the presence/possibility of cell death caused by apoptosis induced in some way by β-ditimonator.
Finally, to test for toxicity effects brought by β-diiminato an acute toxicity evaluation was done on mice. This is a process commonly used to determine adverse effects of a substance after a single or multiple doses. When carried out on mice, the acute toxicity evaluation showed no hepatotoxicity (liver damage) or nephrotoxicity (kidney damage).
So . . .
The study concluded that the β-diiminato compound might trigger apoptosis in human MDA-MB-231 cells, a main player in TNBC. Importantly, animal studies showed no signs of liver or kidney toxicity, supporting the compound’s safety profile. This study highlights β-diiminato as a promising candidate for future anticancer drug design, especially for TNBC treatment while warranting further investigation into its mechanism of apoptosis activation.
While the answers are still unfolding, this research opens new doors in search for more effective and targeted treatments for TNBC.
References
Farghadani, R., Lim, H. Y., Abdulla, M. A., & Rajarajeswaran, J. (2024). Novel indole Schiff base β-diiminato compound as an anti-cancer agent against triple-negative breast cancer: In vitro anticancer activity evaluation and in vivo acute toxicity study. Bioorganic Chemistry, 152, 107730. https://doi.org/10.1016/j.bioorg.2024.107730
Fleury, C., Mignotte, B., & Vayssière, J.-L. (2002). Mitochondrial reactive oxygen species in cell death signaling. Biochimie, 84(2-3), 131–141. https://doi.org/10.1016/s0300-9084(02)01369-x
AI was used for inspiration of the title








