With high specificity, SCOPE’s clinical applications can identify cancer and its growth swiftly. Rapid identification can narrow decisions for a patient’s next steps, innovating cancer screenings.
This newly engineered assay instrument is able to precisely detect genetic mutations within small samples — in just a little over 40 minutes. Done by tackling oncology through testing bodily fluids (liquid biopsies), the diagnostics provides efficient real time insights on a patient’s health.
Using the world renowned gene editing technology CRISPR to elevate the process, SCOPE, the Self-amplified and CRISPR-aided Operation to Profile EVs, is a portable, compact device for benchtop areas.

Researchers from several institutions in Korea and America collaborated on this international project, designing a diagnostic device that sequences messenger RNA (mRNA) from extracellular vesicles (EVs) in liquid biopsies. These EVs are rich in macromolecules, having great potential in clinical settings. Yet technical setbacks such as low abundance of mRNA detected in EVs, have created difficulties in using liquid biopsies due to scarcity within small volume samples.
In the latest study, the research team had developed a streamlined rapid EV mRNA test through addressing issues with pre-amplification, as it brought up arduous tasks such as separate amplification, introducing replication error, and bias. Thus, SCOPE utilizes CRISPR’s Cas13a machinery and signaling template to achieve sensitivity at extremely low concentrations, below attomolar detection limits.
Accessible to All
Medical systemic barriers create challenges in cancer detection, where expertise is required to operate costly equipment. Lab availability and imaging wait times have had a significant impact on the viability of getting early cancer detection. Additionally, diagnostic testing may be unavailable or have restricted access due to lack of trained individuals or an individual’s economic status. Ultimately, there is reduced access to early stage cancer screenings in populations that may have high risk individuals.
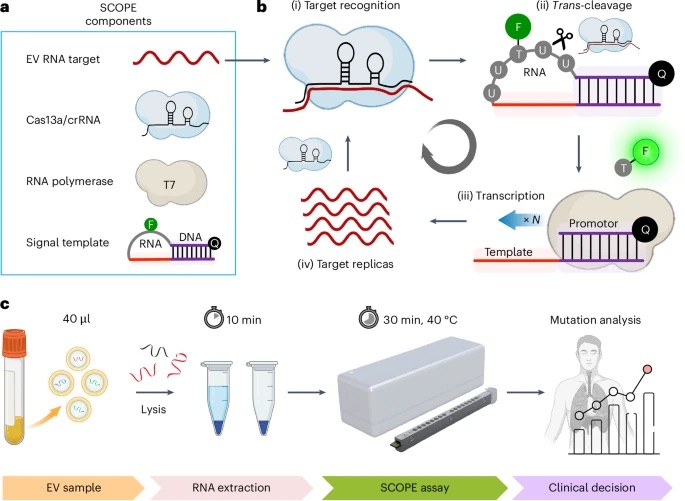
Figure briefly summarizes the SCOPE mechanism (a), the SCOPE assay flow (b), and the SCOPE workflow from sampling to decision (c).
SCOPE addresses accessibility through not only being a small benchtop diagnostic device, it is cost effective at less than $4 a marker in the assay. Additionally through its user-friendliness, it allows clinical technicians to maneuver the assay process through the compact modules engineered by the researchers.
Innovative cancer screening can mitigate cancer fatalities and redirect the future of medical care for individuals with a medical history, cutting out the long wait times.
One-stop Shop
An all-in-one SCOPE mixture is used to introduce liquid samples, this mixture includes CRISPR RNA, T7 polymerase, signal template, and deoxyribonucleotide triphosphates (dNTPs). Essentially, a single step assay optimized for on site testing, without needing to prepare anything in a lab.
Before getting started, the EV is sampled from liquid, such as blood or urine, the EVs are then isolated and lysed. Thereafter the EVs are thrown into surface-treated tubes for RNA extraction, taking approximately 10 minutes.
Once RNA is extracted, a SCOPE reaction occurs in the SCOPE assay in about 3 steps, within 30 minutes. First, a short RNA sequence complementary to the target binds with a Cas protein, making it ready to search and bind to its specific target within the sample. After becoming a ribonuclease complex, the Cas protein is activated and cleaves other nearby nucleic acid molecules in the solution, turning on fluorescent signals from the release of dye molecules. The robust reaction allows the assay to detect the target increase from such nucleic acid presence.
After the fluorescence is identified, clinical decisions can be made based on the diagnostics. The entire process takes less than an hour from sampling to processing data.
Moreover, only one closed tube is needed per sample for the process, making it time efficient and eliminating labour intensity. SCOPE’s assay is recognized as a conservative process by reducing waste compared to other lab processes.
Versatile Detections
The high sensitivity that SCOPE offers was validated through a variety of clinical tests, a few including a mouse model with early-stage lung cancer detection, where blood and plasma samples were used to assess the EVs.
SCOPE has other applications aside from cancer detection, such as the ability to monitor any tumor mutations in patients with colorectal cancer before and after surgery, and categorize brain cancer patients based on genetic profiles from the patient’s peripheral blood.
A typical cancer diagnosis, from screening to results, can range from days to weeks in clinical settings, but SCOPE bridges a diagnostic gap by providing results for same-day clinical decisions within an hour.
Revolutionary Clinical Device
In a time where medical needs have risen with population age and lack of medical professionals, precision in any medical field brings more effective disease diagnosis and accessibility to individuals.
Assay tools like SCOPE, have a wide range of applications with cancer diagnosis, being able to detect early-stage cancers, monitor responses during treatment and stratify patients, this innovates how health professionals may tailor experiences for long term treatments.
As an emerging biomarker, EVs provide an abundance of information on cell health and diseases since they carry biological macromolecules that mirror the state of their parent cells. Analytics on EVs go beyond cancer diagnosis, like providing understanding on intercellular communication or tissue repair to engineer drug delivery.
SCOPE changes the way EVs could be used to work with diagnostics, instead of being neglected in the medical field. Targeted cancer treatments are closer than ever before to a cure.
Citations
Song, J., Cho, M. H., Cho, H., Song, Y., Lee, S. W., Nam, H. C., Yoon, T. H., Shin, J. C., Hong, J.-S., Kim, Y., Ekanayake, E., Jeon, J., You, D. G., Im, S. G., Choi, G.-S., Park, J. S., Carter, B. C., Balaj, L., Seo, A. N., … Lee, H. (2024). Amplifying mutational profiling of extracellular vesicle mrna with scope. Nature Biotechnology, 43(9), 1485–1495. https://doi.org/10.1038/s41587-024-02426-6
(supplementary information on extracellular vesicles)
Kumar, M. A., Baba, S. K., Sadida, H. Q., Marzooqi, S. Al., Jerobin, J., Altemani, F. H., Algehainy, N., Alanazi, M. A., Abou-Samra, A.-B., Kumar, R., Al-Shabeeb Akil, A. S., Macha, M. A., Mir, R., & Bhat, A. A. (2024). Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduction and Targeted Therapy, 9(1). https://doi.org/10.1038/s41392-024-01735-1
No AI was used to write this article.








