Current issues
Cancer is among the most complex and diverse set of diseases to ever plague humanity. New treatments and ideas are tested all the time but there is still much progress to be made. Copper based nanomedicines are one of these ideas being tested, as they can kill cancer cells through a type of cell death called cuproptosis. This is an especially useful lead for cancers which don’t have many good treatments, bringing us to current research on cuproptosis usage against breast cancer.
Breast cancer is the most common form of cancer in the world, and among breast cancers, triple negative breast cancer (TNBC) is the most challenging to treat. One of the main reasons for this is its unique tumor microenvironment (TME), which is stiff and suffocates blood vessels, hurting the immune systems ability to work in the area. Additionally, cancer stem cells (CSCs) are fostered in the TME. These are the commanders of the tumor, which play roles in tumor growth and invade other parts of the body to create new tumors.
Previous copper nanomedicines, such as copper-diethyldithiocarbamate (CuET), showed promise at inducing cuproptosis in cancer cells, but their efficacy in killing CSCs was unknown. This in addition to other physical properties of CuET such as short half life in the bloodstream lead researchers to create and test better nanomedicines which can be delivered to tumors more effectively. The end result is called CuET@PHF which has many advantages compared to its predecessors.
Nanomedicine synthesis
CuET was stabilized with hydroxyethyl starch (HES), polymerized with dopamine, then decorated with folic acid and mercapto group modified HES. HES improves the stability of CuET in aqueous solution, while dopamine increases stability in physiological environments such as those within cancer cells. The folic acid and mercapto groups give the nanomedicine active targeting ability, meaning that they act like heat seeking missiles for cancer cells. Cancers often exhibit a much higher level of folate receptors, which makes folic acid effective at targeting tumors as it binds to the receptors. The mercapto group allows the drug to form disulfide bonds, which are broken only in cancer cells since they have a higher concentration of reduction substances. Release of the drug is therefore controlled and selective to cancer cells.
Treatment of cancer
These properties normally cause cuproptosis in cancer cells, but in the oxygen deprived TME of TNBC, the cuproptosis protein FDX1 is downregulated. This effectively gives the cancer a shield against cuproptosis, so mild photothermal therapy was used. Mild photothermal therapy is the usage of light to heat up tumors. This softens the tumor and improves blood flow within the area, increasing the effectiveness of the immune system in the area and making the TME more normal. The combination of photothermal therapy and nanomedicine was effective in suppressing tumor growth, recurrence, and spread. It should be noted though that the penetration depth of lasers poses a limitation as deeper tumors can’t really be targeted.
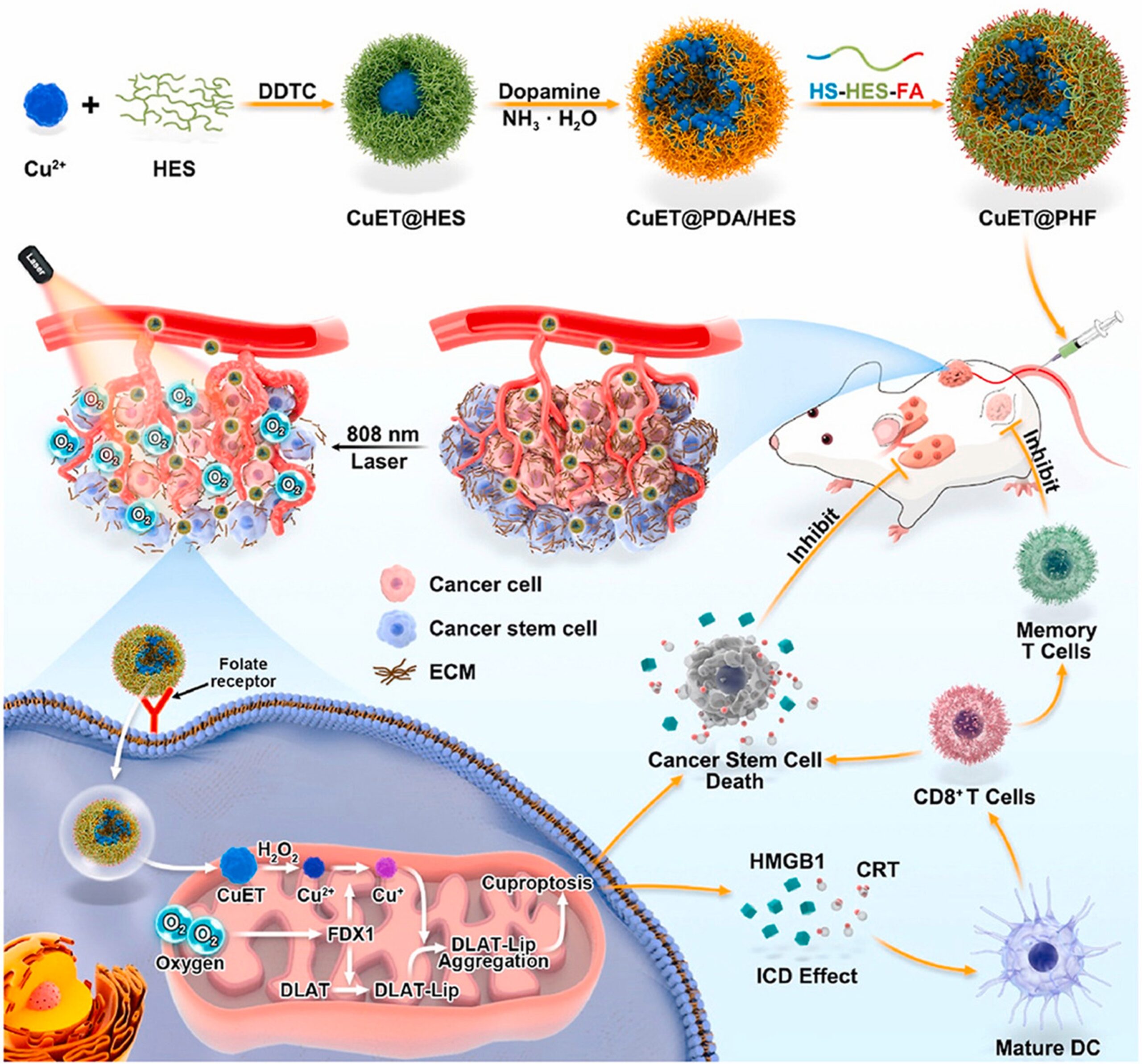
Figure 1: CuET@PHF is created in three steps, delivered via bloodstream into model mice, and combined with mild photothermal therapy to induce cuproptosis in 4T1 cell line CSCs. Immunogenic cell death (ICD) is observed, which involves the release of chemicals that attract the immune system to the area. An anti-tumor immune response is then established which further suppresses the tumor.
Conclusion:
CuET@PHF shows promise in treating TNBC when combined with mild photothermal therapy. Additionally, this study provides a mechanism for how hypoxia downregulates FDX1 and inhibits the effectiveness of cuproptosis. Further animal and clinical experiments could be done to validate effectiveness and safety, as all ingredients used to make CuET have excellent biosafety. Other methods of disrupting hypoxia like oxygen delivery strategies could be researched. Additionally, combination therapy could be tested, such as with immune checkpoint inhibitors.
Cuproptosis has only been known for a few years, with more improvements and clinical tests, cuproptosis based nanomedicines may be an effective tool in the arsenal against cancers. In the next few years, we will likely see if these treatments are the answer, or a dead end.
References
Galluzzi, L., Vitale, I., Warren, S., Adjemian, S., Patrizia Agostinis, Aitziber Buqué Martinez, Chan, T. M., Coukos, G., Demaria, S., Deutsch, E., Dobrin Draganov, Edelson, R. L., Formenti, S. C., Jitka Fucikova, Gabriele, L., Gaipl, U. S., Gameiro, S., Garg, A. D., Golden, E. B., & Zhang, H. (2020). Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. Journal for ImmunoTherapy of Cancer, 8(1), e000337–e000337. https://doi.org/10.1136/jitc-2019-000337t
Guan, J., Wu, Y., Liu, X., Wang, H., Ye, N., Li, Z., Xiao, C., Zhang, Z., Li, Z., & Yang, X. (2021). A novel prodrug and its nanoformulation suppress cancer stem cells by inducing immunogenic cell death and inhibiting indoleamine 2, 3-dioxygenase. Biomaterials, 279, 121180–121180. https://doi.org/10.1016/j.biomaterials.2021.121180
M.D, C. Y. (2024, May 21). Triple-negative breast cancer: 7 things you should know. MD Anderson Cancer Center. https://www.mdanderson.org/cancerwise/triple-negative-breast-cancer-5-things-you-should-know.h00-158986656.html
Wang, P., Chen, B., Zhan, Y., Wang, L., Luo, J., Xu, J., Zhan, L., Li, Z., Liu, Y., & Wei, J. (2022). Enhancing the Efficiency of Mild-Temperature Photothermal Therapy for Cancer Assisting with Various Strategies. Pharmaceutics, 14(11), 2279. https://doi.org/10.3390/pharmaceutics14112279
Xiao, C., Wang, X., Li, S., Zhang, Z., Li, J., Deng, Q., Chen, X., Yang, X., & Li, Z. (2024). A cuproptosis-based nanomedicine suppresses triple negative breast cancers by regulating tumor microenvironment and eliminating cancer stem cells. Biomaterials, 313, 122763–122763. https://doi.org/10.1016/j.biomaterials.2024.122763
AI Use Statement:
AI was used to clarify some technical concepts including immunogenic cell death and the role of folic acid and mercapto groups in CuET@PHF