With an ever growing population and limited resources on our planet, sustainability is essential for future development. This serves as the basis for researches conducting experiments on ways of producing meat alternatives, aiming to find better ways of cultivating meat that is both environmentally friendly as well as delicious. While there are meat alternatives currently available, including plant-based proteins that aim to replace meat as the primary source of protein in a diet, they lack the texture, flavor, and mouthfeel of real meat. Lab grown meat has shown promise in terms of development and may be a breakthrough for our future generations’ diets. Some studies have already been conducted, including the creation of bovine cell fiber-based hamburgers, and other various types of cultured meat. However producing lab-grown steak proves to be a challenge due to its composition.
Tissue engineering is the method by which we take the cells and eventually make cultured meat, and it includes cell sheet engineering, cell fiber engineering, cell culture on 3D-printed scaffold, and 3D cell printing. The structural characteristics of steak were most likely to be mimicked by 3D cell printing, as the controllability of the output is better compared to the other technologies.
Stem cells are special types of cells that have the ability to grow and differentiate into other types of cells with specific purposes. They are a key ingredient for the success of lab grown meat. In particular, bovine stem cells (bSCs) and bovine adipose-derived stem cells (bADSCs) originating from cows were used to culture meat. These cells are precursors to what will eventually become the steak-like meat consisting of the same muscle, fat, and vascular tissues (blood vessels) that traditional steaks have.
So how do we go from cells to steak?
Steak is comprised of muscle tissue, fat tissue, and vascular tissue. To obtain these specific types of tissues, researchers first had to confirm the cells’ proliferation and differentiation as there is not much pervious research on this topic. Researchers studied the different stem cells’ differentiation into the appropriate cell types by growing these cells in controlled conditions (culturing), including a specific medium that aids in the cells growth and multiplication. The special medium containing fetal bovine serum, basic fibroblast growth factor helps the bSCs’ ability to grow and multiply. After a certain time, the cells are transferred to a differentiation medium containing horse serum, which is used to induce differentiation into muscle cells.
Fat cells are also needed for the engineered steak. To make these, bADSCs are what we need to start with. Researchers found that a specific mix of seven free fatty acids as well as an inhibitor of the TGF-β known as ALK5i was good for inducing lipogenesis (fat formation). The bADSCs are also used to differentiate into endothelial cells, the cells that line our blood vessels. By using horse serum, endothelial differentiation of bADSCs is encouraged, which could have potential uses not in just engineered steak and meat, but other regenerative medicines as well.
Now that the different types of cells have been cultured, the assembly of all these cells together is carried out by a special type of 3D printing. The technology used is called supporting bath-assisted 3D printing (SBP), and uses special bioink containing stem cells that are printed into a supporting bath. In this study, the bath was composed of gelatin and gellan gum as they were easy to deal with and remove after the printing process was completed. The bath holds the cells in place which can sustain the printed structure for longer durations if needed, as well as keeping the structure intact.
At this point in the study, the researchers had to independently fabricate each cell type separately, but it is proposed that a differentiated media for culturing all 3 types of cell fibers could be developed to streamline the process and make this technology more feasible.
An issue with SBP is that, over time, the fibers would collapse into a globular form.

Figure 3C, showing the fibers after printing (above) and how they fold into globular shapes (below)
To address this, the researchers fixed the ends of the fibers, encouraging alignment of them, leading to the modification of SBP with tendon-gel-integrated bioprinting (TIP). TIP was developed in this study, and introduces 2 layers of tendon gels which serve as anchor points for the cell fibers. The result was a well-maintained fibrous structure and cell alignment, which closely resembles the structure of real meat.
Putting all these technologies together, we can assemble the TIP-derived cell fibers together to make an engineered steak. The researchers did this by producing a cultured steak approximately 5mm x 10mm x 5mm in size, consisting of 42 muscle cell fibers, 28 fat cell fibers, and 2 blood capillary cell fibers.
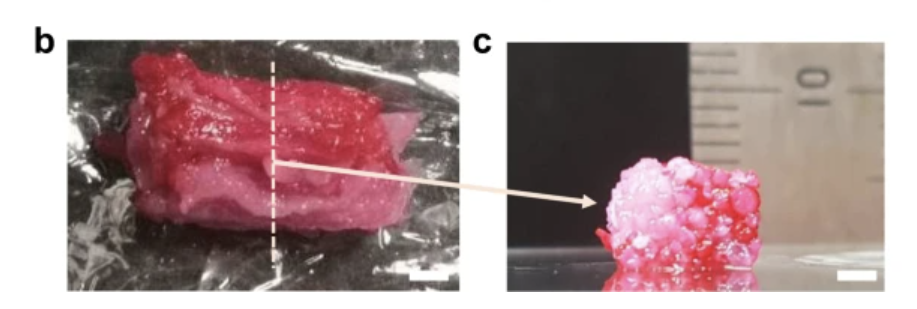
Figure 5B,C, showing the engineered steak final product of the study (left), and a cross section (right)
In conclusion, creating engineered steak has proven to be a challenge that the researchers have addressed with a good effort, demonstrating that 3D printing holds promise for the development of cultured meats. By harnessing the power of stem cells and advanced 3D bioprinting techniques, the possibility of steak from a lab is becoming a reality, paving a way for more sustainable food future. As this technology continues to develop, we could eventually enjoy meats without having to worry about the environmental impacts, and even ethical concerns with traditional meat production and consumption.