The main obstacle to studying human brain development is a lack of access to brain tissue. Today, scientists can stimulate stem cells to form human cortical organoids (hCOs), tiny self-organized tissue cultures that resemble the brain. Although hCOs can be a useful tool to model brain development, they are limited by the in vitro environment. “In vitro” means outside of an organism, whereas “in vivo” means inside an organism.
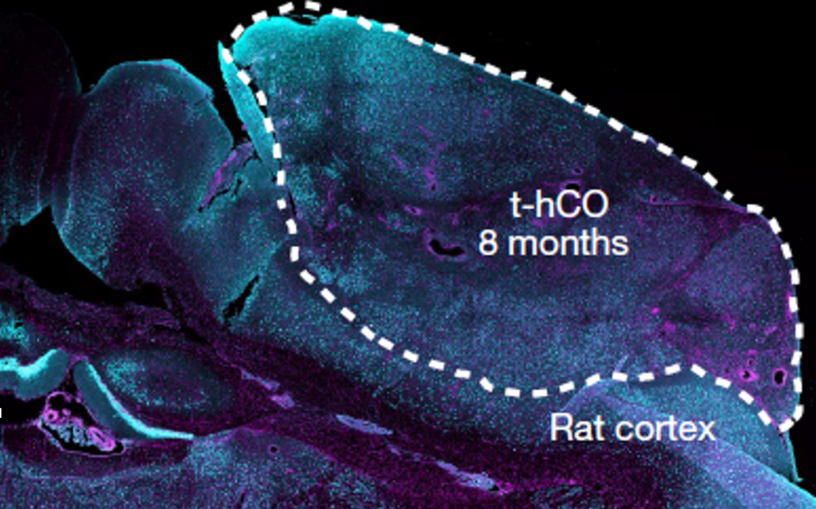
Specifically, it is unclear whether hCO maturation is restricted by the absence of sensory inputs and other cells found in vivo. Furthermore, hCOs cannot integrate into circuits that generate behavioural outputs, limiting their capacity to model neuropsychiatric diseases. To overcome these limitations, researchers from Stanford transplanted hCO into the brains of newborn rats to assess how transplanted hCOs (t-hCOs) matured and integrated into neural circuits.
Previous studies have demonstrated that human neurons transplanted into rodent brains can survive and form connections with other cells. However, these experiments were performed in adult rodents. Researchers predicted that t-hCOs would integrate better into newborn rats since their brains are in an early developmental stage.
First, researchers generated hCOs from human stem cells and transplanted hCOs into newborn rats. They used magnetic resonance imaging (MRI) and antibody staining techniques to ensure t-hCOs were growing within the rat brain. Next, the researchers conducted a series of transcriptional, morphological, and functional analyses to compare in vivo t-hCO neurons and in vitro hCO neurons. They found that t-hCO showed greater maturation compared to hCOs. For instance, t-hCO neurons were larger and fired at higher maximal rates than hCO neurons. Single-nucleus RNA sequencing revealed that t-hCO also had a more mature transcriptome with upregulation of genes associated with maturation.
Through these analyses, researchers found that t-hCOs closely resembled human neurons in the late fetal phase. This prompted them to investigate whether t-hCO could be used to study human diseases. hCO was generated from three patients with Timothy Syndrome (TS), a severe neurodevelopmental disease. Researchers discovered that TS t-hCO neurons had twice as many dendrites and reduced dendrite length, compared to healthy neurons. Furthermore, TS t-hCO neurons had an abnormal dendritic branching pattern that was not observed within in vitro TS hCOs. This highlighted the ability of t-hCOs to reveal disease phenotypes within an in vivo system.
Researchers then examined how t-hCO cells functionally integrated into the rat brain. Antibody staining identified the presence of thalamic terminals in t-hCO which are associated with receiving sensory input. To assess whether t-hCO could be activated by sensory stimuli, scientists performed an experiment where they deflected the rat’s whiskers and measured neuronal activity with two-photon calcium imaging. They found t-hCO activity increased in response to whisker deflection, indicating that t-hCO can receive sensory inputs and respond to environmental stimuli.
Finally, the scientists investigated whether t-hCO can modify behaviour. Researchers used optogenetics, a technique that uses light to stimulate neuron activity, to see how rats modified their response to stimuli when a reward is present. In this experiment, rats were transplanted with an opsin-expressing hCO and an optogenetic fibre that delivers light to the brain. Opsin is a protein that senses light and activates the cell during light stimulation. Rats received a water reward if they licked during the blue light stimuli, but they did not receive a reward during the red light stimuli. By the fifteenth day, animals transplanted with opsin-expressing hCO showed increased licking during blue light stimulation, compared to the control group. This suggests t-hCO cells can activate rat neurons to drive reward-seeking behaviors. Immunohistochemistry analysis further revealed activation of brain regions associated with motivated behaviors by identifying FOS, an activity-dependent protein.
In conclusion, these results showed that t-hCOs could overcome many of the limitations associated with hCOs in vitro. t-hCO showed enhanced maturation and integrated into circuits that respond to sensory inputs and modulate behaviour. In comparison to past studies that transplanted human cells into rodent brains, the researchers’ use of newborn rats likely favoured greater integration of t-hCOs, and their use of MRI to monitor t-hCO growth allowed them to perform longer-term studies across multiple animals. Overall, t-hCOs are a promising tool to study brain development that will hopefully help us uncover new disease phenotypes and test new therapeutic strategies.
Source: Revah O, Gore F, Kelley KW, Andersen J, Sakai N, Chen X, et al. Maturation and circuit integration of transplanted human cortical organoids. Nature. 2022 Oct 13;610(7931):319–26.